Peroxide ion: All you need to know about the chemical compound
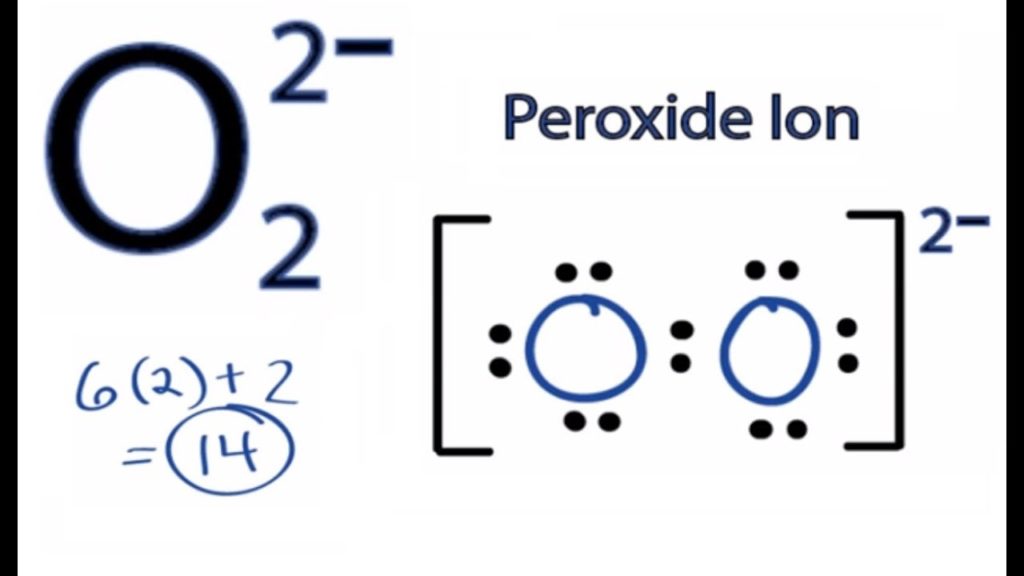
The peroxide ion, O22−, has a solitary oxygen-oxygen covalent bond and an oxidation condition of −1 on the oxygen particles. The peroxide ion is an amazing hydrogen ion acceptor, making the peroxides of the antacid metals and soluble earth metals solid bases.
Peroxides are a gathering of mixtures with the construction R−O−O−R, where R = any element.[1][2] The O−O bunch in peroxide is known as the peroxide bunch or peroxo bunch. The terminology is to some degree variable.[3]
The most well-known peroxide is hydrogen peroxide (H2O2), informally referred to just as “peroxide”. It is showcased as solutions in water at different concentrations. Numerous natural peroxides are known too.
Construction and dimensions of H2O2
Beside hydrogen peroxide, some other significant classes of peroxides are:
Peroxy acids, the peroxy subordinates of numerous recognizable acids, models being peroxymonosulfuric corrosive and peracetic corrosive, and their salts, one illustration of which is potassium peroxydisulfate.
Peroxide, any of a class of synthetic mixtures wherein two oxygen molecules are connected together by a solitary covalent bond.
A few natural and inorganic peroxides are valuable as dying specialists, as initiators of polymerization reactions, and in the preparation of hydrogen peroxide (q.v.) and other oxygen compounds.
The contrarily charged peroxide ion (O22-) is available in inorganic mixtures that might be viewed as salts of the exceptionally powerless corrosive hydrogen peroxide; models are sodium peroxide (Na2O2), a fading specialist, and barium peroxide (BaO2), once in the past utilized as a wellspring of hydrogen peroxide.
Two classifications of peroxides exist in which either of the oxygen particles are covalently connected to iotas other than hydrogen.
One class is addressed by cumene hydroperoxide, a natural compound utilized as a polymerization initiator and as a wellspring of phenol and CH3)2CO, and peroxysulfuric corrosive, an inorganic compound utilized as an oxidizing specialist.
The other classification incorporates di-tert-butyl peroxide and ammonium peroxydisulfate, both utilized as initiators.
Hydrogen Peroxide
Hydrogen peroxide is a substance compound with the equation H2O2 in its unadulterated structure, it is an exceptionally pale blue fluid, somewhat more gooey than water.
It is utilized as an oxidizer, blanching specialist, and clean, typically as a weakened solution (3–6% by weight) in water for purchaser use, and in higher concentrations for mechanical use. Concentrated hydrogen peroxide, or “high-test peroxide”, deteriorates violently when warmed and has been utilized as a charge in rocketry.
Hydrogen peroxide is a responsive oxygen animal group and the least complex peroxide, a compound having an oxygen–oxygen single bond. It deteriorates gradually when presented to light, and quickly within the sight of natural or responsive mixtures.
It is ordinarily put away with a stabilizer in a pitifully acidic solution in a dim container to hinder light. Hydrogen peroxide is found in organic frameworks including the human body. Catalysts that utilize or deteriorate hydrogen peroxide are delegated peroxidases.
Peroxides
Peroxides fix by warm decomposition into oxy revolutionaries, which unique hydrogen from the elastomer to create polymer extremists. These then, at that point, respond to shape carbon-carbon crosslinks.
With unsaturated elastomers, this happens especially at the site of allylic hydrogens. The pace of crosslinking is straightforwardly proportional to the pace of decomposition of the peroxide.
Fix rates and restoring temperatures thusly rely upon the steadiness of the peroxide, which diminishes in the request dialkyl > perketal > perester or diaryl. The most normally utilized of these crosslinkers is dicumyl peroxide.
Despite the fact that carbon-carbon crosslinks are more thermally stable than sulfur crosslinks, they give commonly less fortunate malleable and tear strength.
Peroxides are additionally incongruent with a large number of the cell reinforcements utilized inelastic since the cancer prevention agents are intended to search and obliterate oxy and peroxy-free revolutionaries (see Antioxidants, underneath).
Peroxides can’t be utilized with butyl elastic since they cause chain scission and depolymerization.

Peroxide fixes
Peroxide fixes can be depicted as free extreme fixes started by the warm decomposition of peroxide. Endless supply of a free extreme oxygen animal type, hydrogen is disconnected from the polymer chain.
The subsequent carbon revolutionary species then, at that point, responds with another free extremist (perhaps on another polymer chain), responds with one more twofold attach to create another free extreme species (and potentially a crosslink), or starts a degradation cycle of the polymer chain breaking.
(The degree to which every one of these various reactions happens is reliant upon the substance construction of the elastomer.)
The net aftereffect of this interaction is to produce carbon-carbon connections between polymer chains. The temperature at which the peroxide disintegrates and the pace of the generation of the free extreme species are reliant upon the design of the peroxide utilized.